没有合适的资源?快使用搜索试试~ 我知道了~
The puzzle of PCNA's many partners
需积分: 11 0 下载量 42 浏览量
2021-06-29
19:43:31
上传
评论
收藏 499KB PDF 举报
温馨提示
The puzzle of PCNA's many partners The puzzle of PCNA's many partners Emma Warbrick Summary The identification of proteins that interact with proliferat- ing cell nuclear antigen (PCNA) has recently been a rapidly expanding field of discovery. PCNA is involved in many aspects of DNA replication and processing, form- ing a sliding platform that can mediate the interaction of proteins with DNA. It is striking that many proteins bind to PCNA through a small region containing a conserved motif; t
资源推荐
资源详情
资源评论

The puzzle of PCNA's many partners
Emma Warbrick
Summary
The identification of proteins that interact with proliferat-
ing cell nuclear antigen (PCNA) has recently been a
rapidly expanding field of discovery. PCNA is involved in
many aspects of DNA replication and processing, form-
ing a sliding platform that can mediate the interaction of
proteins with DNA. It is striking that many proteins bind to
PCNA through a small region containing a conserved
motif; these include proteins involved in cell cycle
regulation as well as those involved in DNA processing.
Sequential and regulated binding of motif-containing
proteins to PCNA may contribute to the ordering of events
during DNA replication and repair. Results from bacterio-
phages and archaea show that the structural basis for the
interaction of this motif with PCNA is extremely ancient.
The analysis of how such functional motifs have been
recruited to proteins in present day organisms helps us to
understand how these complex systems arose from
ancestral organisms. BioEssays 22:997±1006, 2000.
ß 2000 John Wiley & Sons, Inc.
Introduction
PCNA (proliferating cell nuclear antigen) was originally dis-
covered as an antigen found only in the nucleus of dividing
from which it derives its name.
(1)
It was independently iden-
tified as a protein with elevated levels during S phase of the cell
cycle.
(2)
A few years later, as a result of work to reconstitute
SV40-dependent replication in vitro, PCNA was identified as
an essential factor in DNA replication.
(3)
PCNA is now known
to be essential for the processivity of the DNA polymerase
complex, and also for the correct co-ordination of leading and
lagging strand synthesis.
(4)
Study of the crystal structure of
PCNA reveals a ring-shaped trimeric complex with striking
sixfold symmetry, which can encircle double-stranded DNA
and slide freely along it.
(5,6)
This structural information was
the vital clue to how PCNA could stably associate with DNA
without binding directly to it, and how it could link the poly-
merase complex to the DNA strand in a processive manner. It
is now known that, although PCNA has no endogenous enzy-
matic activity, it forms a sliding platform that can mediate the
interaction of several proteins with DNA in a non-sequence-
specific manner.
(7±9)
So-called ``sliding clamps'' are highly
conserved through evolution: homologues of PCNA are found
in eukaryotes, archaea, bacteriophages and some viruses.
The b-subunit of E. coli DNA polymerase III shares a very
similar three-dimensional structure with eukaryotic PCNA,
despite forming a dimeric, rather than trimeric, complex.
In the last few years, it has been shown that PCNA is
essential not only for DNA replication, but also for several
forms of DNA repair, including nucleotide excision repair
(NER), base excision repair (BER) and mismatch repair
(MMR).
(7,8)
More recently, a role for PCNA has been demon-
strated in various aspects of post-replicative processing, such
as cytosine methylation and chromatin assembly, although the
exact function of PCNA in these processes is not clear.
The identification of proteins that interact with PCNA has
been extensively studied recently.
(10)
As predicted, many
PCNA-interacting proteins are involved in various aspects of
DNA replication and processing, and hence these proteins are
probably using PCNA's ``sliding clamp'' properties to mediate
their interaction with DNA. It is intriguing, however, that cell
cycle regulatory proteins have also been found to interact with
PCNA. Such proteins, which include p21
WAF1/Cip1
, p57, cyclin
D, Gadd45 and Myd118 play multiple and, in many cases, not
completely understood, roles in the regulation of proliferation
and cell cycle progression. Although there is no clear evidence
yet, it seems likely that protein interactions with PCNA may be
a mechanism to co-ordinate DNA replication and repair with
the cell cycle.
One of the most interesting observations that has emerged
from the study of PCNA-binding proteins is that many contain
a conserved PCNA-binding motif (Table 1; Figure 1).
(11)
The PCNA-binding motif present in the regulatory protein p21
(also known as WAF1, Cip1, Sdi1 etc.) has been extensively
characterised, and its interactions with PCNA have been
mapped at the molecular level.
(6,12)
Biochemical evidence has
shown that other motif-containing proteins share a common
binding site on PCNA.
(13±17)
Although each PCNA trimer
theoretically has the capacity to interact with three such
proteins, the large number of motif-containing proteins far
exceeds this capacity, suggesting that the interactions of these
proteins with PCNA must be regulated. As described above,
this motif is also found in regulatory molecules, such as p21,
which have roles in regulating proliferation and in DNA
BioEssays 22:997±1006, ß 2000 John Wiley & Sons, Inc. BioEssays 22.11 997
Department of Surgery and Molecular Oncology, University of
Dundee, Ninewells Hospital and Medical School, Dundee DD1 9SY,
UK. E-mail: e.warbrick@dundee.ac.uk
Funding agency: The Association for International Cancer Research.
Abbreviations: ATP, adenosine triphosphate; BER, base excision
repair; CDK, cyclin-dependent kinase; CKII, casein kinase II; DNA
Mtase, DNA cytosine 5-methyltransferase; MMR, mismatch repair;
NER, nucleotide excision repair; PCNA, proliferating cell nuclear
antigen; RFC, replication factor C; RPA, replication protein A; UDG,
uracil DNA glycosylase.
Review articles
damage checkpoint control. This suggests that the co-
ordinated binding of proteins via the conserved PCNA-binding
motif may provide a regulatory mechanism to co-ordinate
aspects of DNA metabolism, such as replication and repair,
within the cell cycle. It should be emphasized, however, that
several PCNA-binding proteins do not appear to interact
through such a conserved motif. These include the regulatory
protein Gadd45, which also binds to p21 and may be involved
in regulating DNA repair, and CAF1, which links chromatin
assembly to DNA replication through its interaction with
PCNA.
(18±21)
In this article, I will review the eukaryotic proteins that have
been identified as binding to PCNA through the conserved
motif, put forward some speculation about the evolutionary
history of this motif, and discuss how the motif may be involv-
ed in co-ordinating the protein function through PCNA
interactions.
PCNA-interacting proteins:
cell cycle control
The PCNA-binding motif was first identified in p21
WAF1/Cip1
, a
protein that can be transcriptionally regulated by p53 and
which interacts with cyclins and cyclin-dependent kinases
(CDKs). The PCNA-binding region of p21 has been exten-
sively characterised and the structure of a p21-derived peptide
co-crystallised with human PCNA has been determined.
(6,12)
This peptide has a complex interaction with PCNA, with each
PCNA trimer having three potential peptide-interaction sites.
During the interaction, the peptide makes contacts with three
distinct regions of the PCNA surface: (1) the C-terminal region
makes a b-sheet interaction with the interdomain loop that
joins the two structural domains of the PCNA monomer, (2) the
three central residues of the peptide are anchored in a
hydrophobic pocket, and (3) residues from the N terminus of
the peptide form poorly ordered interactions with the C
Table 1. Eukaryotic proteins containing the conserved PCNA-binding motif
Protein Function PCNA-binding consensus
p21 CDK regulatory protein
RQ--MTDFY- - -RR- - -
Fen1 Structure specific endonuclease -QGRLD-FFK- --S---
Cdc27 (p66) DNA polymerase d subunit -Q--I-SFF- -K
DNA ligase I Replication-specific DNA ligase -Q--I--FF- - -K- -K-
RFC p140 Large subunit of PCNA-loading complex -I--FFG- - - - - -K
MSH3 Mismatch repair protein -Q--LSRFF- - - - - ---
MSH6 Mismatch repair protein -Q--L-SFF-K- - - - --
XPG Nucleotide excision repair endonuclease TQ-RI- -FF- - - - - - - -
MCMT 5
0
cytosine methyltransferase -Q-TI- -HF- - - - -KRK
UNG2 Nuclear form of UNG uracil DNA glycosylase -Q-TL- -FF- - - - - - - -
WRN Helicase required for genomic stability DQWKL- -DF- -KL- - - -
POGO Type II transposases -Q--L--FY
Highly conserved residues known to make essential contacts in the p21-PCNA structure are shown in bold. Where significant homologies between
homologues exist, these residues are shown.
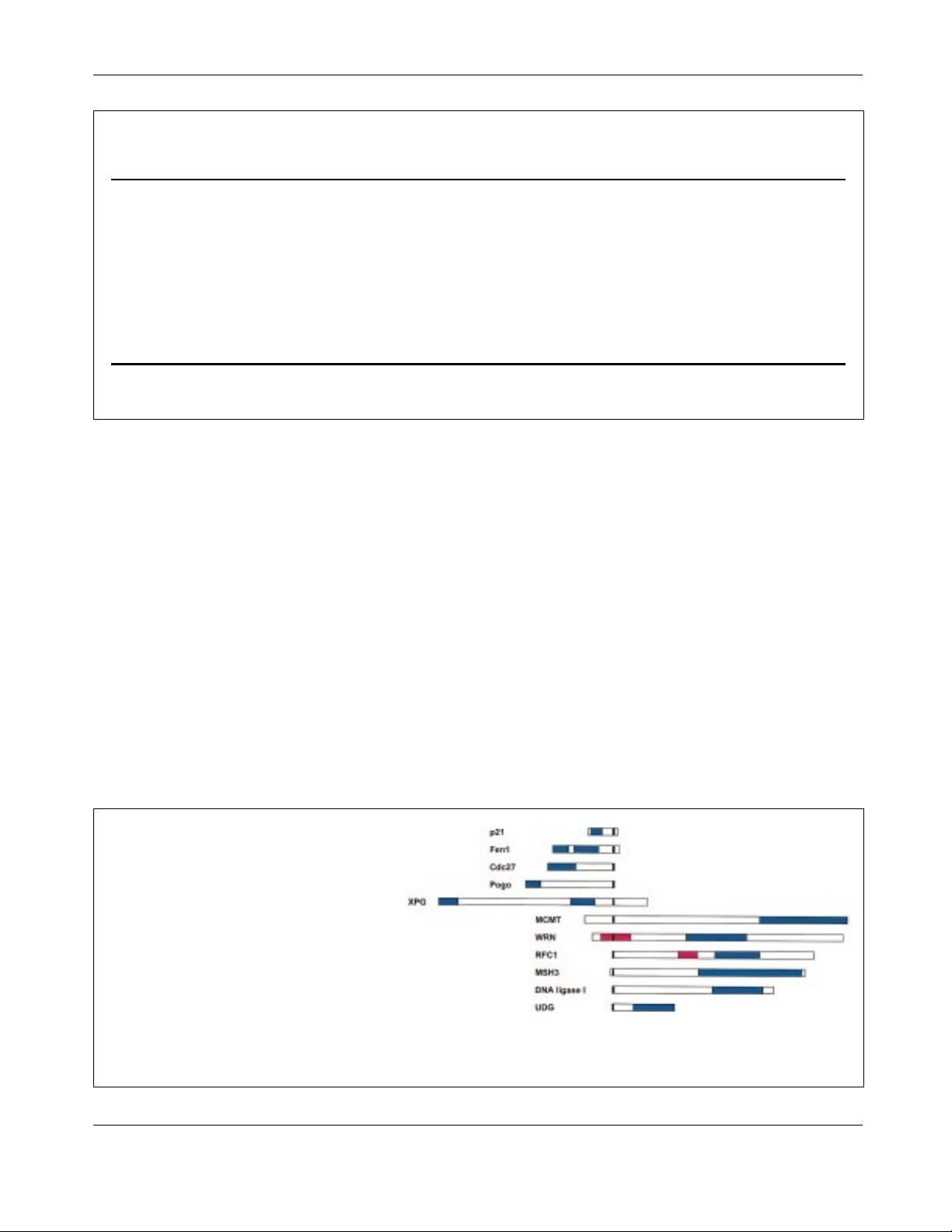
Figure 1. A schematic representation of PCNA-
binding motifs within eukaryotic proteins. The black
box denotes the position of the PCNA-binding
domain, while coloured boxes indicate other
functional domains. Representations are based
on the human protein, unless stated otherwise.
The coloured domains are defined as follows: p21,
CDK/cyclin-binding domains by similarity to
p27Kip1;
(86)
Fen1 and XPG, endonuclease do-
mains (by similarity); Cdc27 (S. pombe), Cdc1-
binding domain;
(31)
Pogo (Drosophila), DNA-bind-
ing domain of the transposase;
(87)
DNA ligase I,
the catalytic domain based on sequence similar-
ity;
(88)
MCMT, methyltransferase domain;
(89)
WRN, exonuclease domain shown in red, helicase domain in blue;
(90)
RFC, DNA-binding
domain in red, PCNA-interacting domain in blue;
(40)
MSH3, the coloured domain shows a region of sequence of similarity to other
mismatch repair proteins from information in the Prodom database; UNG, catalytic domain based on studies of sequence similarity.
Review articles
998 BioEssays 22.11
剩余9页未读,继续阅读
资源评论

weixin_38684892
- 粉丝: 10
- 资源: 936
上传资源 快速赚钱
 我的内容管理
展开
我的内容管理
展开
 我的资源
快来上传第一个资源
我的资源
快来上传第一个资源
 我的收益 登录查看自己的收益
我的收益 登录查看自己的收益 我的积分
登录查看自己的积分
我的积分
登录查看自己的积分
 我的C币
登录后查看C币余额
我的C币
登录后查看C币余额
 我的收藏
我的收藏  我的下载
我的下载  下载帮助
下载帮助

 前往需求广场,查看用户热搜
前往需求广场,查看用户热搜最新资源
- 2015-2024年上市公司商道融绿esg评级数据(年度)
- DeepSeek:通用人工智能从入门到精通的技术解析与应用指南
- 离散扩展龙伯格观测器:扰动补偿功能下的鲁棒性能优化及动态响应增强策略,离散扩展龙伯格观测器:具有扰动补偿功能的高鲁棒性预测控制系统,一种具有扰动补偿功能的离散扩展龙伯格观测器,有较好的参数摄动扰动抑制
- 无刷直流电机BLDC三闭环控制系统的Matlab Simulink仿真模型搭建:原理、波形记录与参数详解,无刷直流电机BLDC三闭环控制系统的Matlab Simulink仿真模型搭建:原理、波形记录
- 基于Python的Django-vue基于spark的短视频推荐系统的设计与实现源码-说明文档-演示视频.zip
- DeepSeek写的重力球迷宫手机小游戏
- 单相变压器绕组与铁芯振动形变仿真模型:洛伦兹力与磁致伸缩效应下的动态响应分析,COMSOL单相变压器绕组与铁芯振动形变仿真模型:基于洛伦兹力与磁致伸缩效应的时域分析,comsol的单相变压器绕组及铁芯
- 新兴经济体二氧化碳排放报告2024.pdf
- 激光熔覆技术:COMSOL模拟建模与视频教程服务,助力激光研究人员与工程师的专业提升,激光熔覆技术:COMSOL软件下的建模与视频教程应用指南,COMSOL 激光 激光熔覆 名称:激光熔覆 适用人群:
- 2000-2023年上市公司价值链升级数据(含原始数据+计算代码+结果)
- COMSOL仿真下的钢架无损超声检测:焊接区域及周边缺陷识别技术,角钢梁纵波转横波检测原理揭秘,Comsol仿真技术下的钢架无损超声检测:角钢梁缺陷的精准识别与定位,Comsol仿真钢架无损超声检测
- 基于FPGA的图像坏点像素修复算法实现及Matlab辅助验证:探索其原理、测试与使用视频教程 注:标题中的“可刀”一词在此上下文中并无实际意义,因此未被包含在标题中 标题长度符合要求,并尽量简洁明了
- 2008-2022年各省环境污染指数数据(原始数据+结果).xlsx
- zhaopin_mzhan.apk
- 权威科研机构发布钢轨表面缺陷检测数据集,含400张图像和8种类别缺陷,mAP达0.8,附赠lunwen,钢轨表面缺陷检测数据集:包含400张图片与八种缺陷类别,适用于目标检测算法训练与研究 ,钢轨表面
- C形永磁辅助同步磁阻电机Maxwell参数化模型:转子手绘设计及关键参数优化分析,基于Maxwell参数化模型的C形永磁辅助同步磁阻电机研究:转子手绘非UDP模块的参数化设计及优化分析,C形永磁辅助同
资源上传下载、课程学习等过程中有任何疑问或建议,欢迎提出宝贵意见哦~我们会及时处理!
点击此处反馈



安全验证
文档复制为VIP权益,开通VIP直接复制
 信息提交成功
信息提交成功