838 J. Luo et al. / J. Math. Anal. Appl. 434 (2016) 837–857
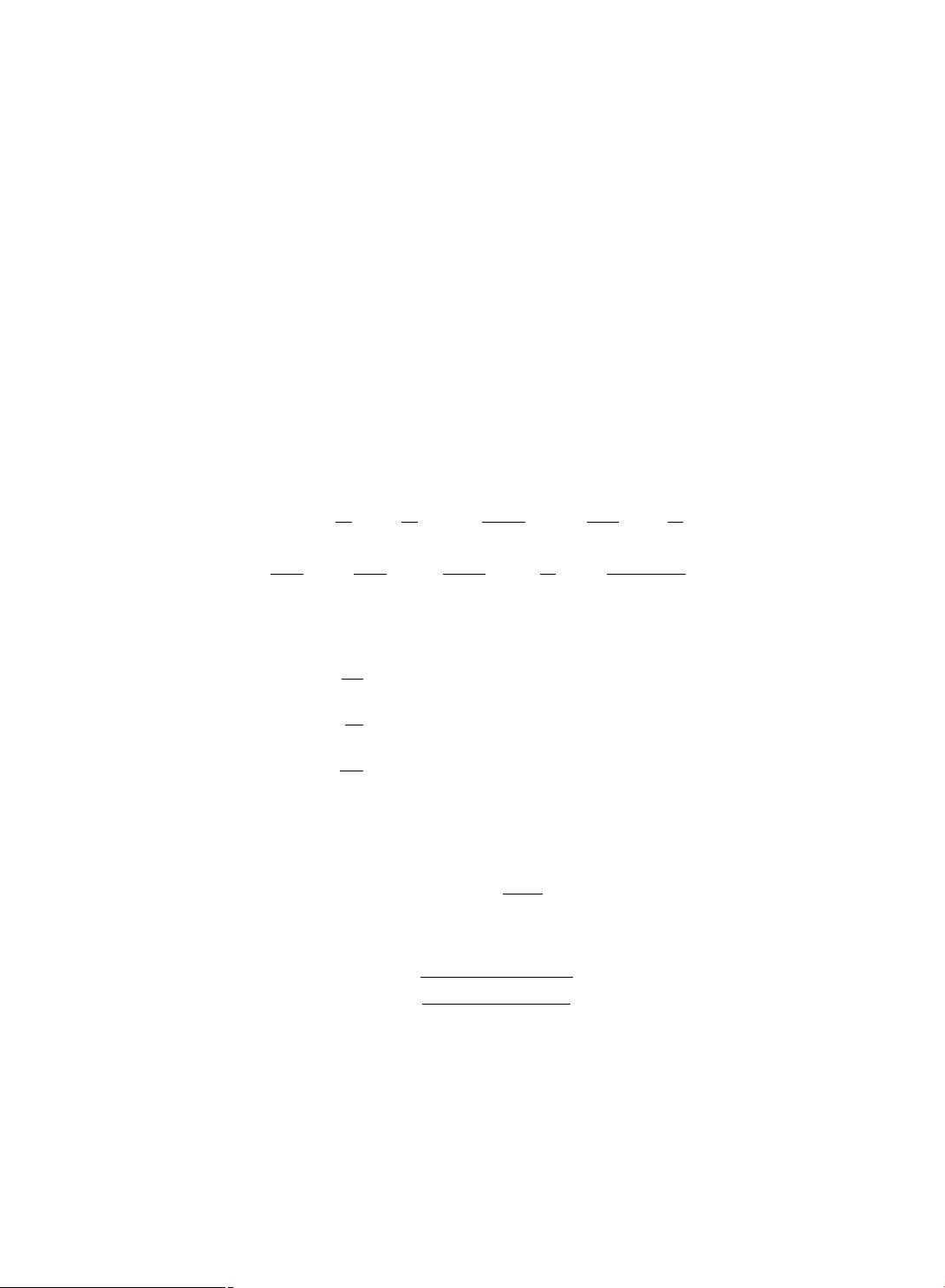
dT
dt
= λ − δ
1
T − βV T,
dI
dt
= βV T − δ
2
I,
dV
dt
= NI − cV,
(1.1)
where T is the concentration of uninfected target cells, I is the concentration of infected cells, V is the
concentration of virus, λ is the production rate of new target cells, δ
1
and δ
2
are the death rates of unin-
fected
cells and infected cells respectively, β is the infection coefficient, N is the burst coefficient of virus
when infected cells die and c is the clearance rate of virus. Model (1.1) has made significant contributions to
understand the dynamics of HIV infection and therapy to design specific treatment regimes [29,46]. How-
ever,
as noted in [29], since immune responses are not explicitly included, model (1.1) is suitable for the
asymptomatic phase of the disease during which immune responses are stable. Indeed, in the primary phase
of HIV infection, the cellular immune response to HIV is strong, grows with the rise in HIV in the blood
and then falls when that response reaches a peak [11,26].
Immune responses have been incorporated in the basic model of HIV infection (see, for example, [1,3,14,
20,21,28,31,32,34,35,43,45,47] and
the references cited therein) to understand how immune systems regulate
the dynamics of HIV infection. Most of these researches study effects of cytotoxic T lymphocyte (CTL)
in fighting HIV replication, and only a few consider the effect of proliferation of CD4
+
T cells under the
stimulation of pathogens. Indeed, biological studies indicate that CD4
+
T cell activation plays a fundamental
role in guiding CTL responses [11,26]. In papers [2,33,48], an HIV model is proposed that incorporates CTL
responses to intracellular pathogens, CD4
+
T cell proliferation due to stimulations of pathogens, and B
cell responses to virus particles. The model admits only three state variables, which makes mathematical
analysis tractable. More importantly, the model captures the main features of the complex interactions of
HIV and immune responses. The dynamics of the variables are described by
⎧
⎪
⎪
⎪
⎪
⎪
⎪
⎨
⎪
⎪
⎪
⎪
⎪
⎪
⎩
dT
dt
=(λ + γ
1
IT)(1 − T/K) − δ
1
T − βV T,
dI
dt
= βV T − (δ
2
+ γ
2
T )I,
dV
dt
= NI − (c + γ
3
T )V −βV T.
(1.2)
Here, T is the concentration of helper T cells, I is the concentration of infected helper T cells, V is the
concentration of free virus. Parameter γ
1
represents the clonal amplification of helper T cells after stimulation
by infected T cells. Further, the recruitment rate and the proliferation rate of helper T cells are limited
by the carrying capacity K. The model assumes that B cell and CTL responses are proportional to the
concentration of helper T cells. As a consequence, term γ
2
TI gives the removal rate of infected cells by CTL
responses and term γ
3
TV denotes the clearance rate of virus particles by antibodies. The last term −βV T
describes the loss rate of virus because of entry into target cells. All the other parameters in (1.2) have the
same meaning as those in (1.1).
Numerical simulations of model (1.2) in [2] found the case of asymptotic coexistence of immune system
and virus, and found the case of virus eradication. The stability of infection-free equilibrium of (1.2) was
examined in [48]. Model (1.2) was also extended by [48] to include stochastic cellular reproduction and
death, stochastic infection process, stochastic immune system activation, and stochastic viral reproduction.
In this paper, we explore the global behaviors of model (1.2) to find possible evolutionary outcomes of
HIV infection under the control of immune responses. We establish conditions for the global stability of
infection-free equilibrium and infection equilibrium, which gives the conditions of infection eradication or