
N
eurons in the neocortex consist of two broad classes:
glutamatergic excitatory principal neurons and g-amino-
butyric acid (GABA)-ergic inhibitory interneurons.
They form intricate neuronal networks for information
processing and behavioural control. While excitatory neurons
account for the vast majority of the neuronal population and are
largely responsible for information flow and neural computation,
inhibitory interneurons are an integral part of functional
circuits and provide a rich variety of synaptic inhibitions to
shape neuronal activity and circuit operation
1–4
. To understand
the operation and function of the neocortex, it is crucial to
decipher the precise connectivity of neocortical neurons. Much of
the effort has focused on excitatory neurons, which exhibit
remarkable precision in synaptic connectivity and functional
organization. In general, excitatory connections respect laminar
and columnar functional architectures, and conform to ‘canonic’
organization
5–7
. In comparison, our understanding of the
circuit organization of inhibitory interneurons in the neocortex
remains limited.
While a great degree of specificity in the subcellular
synaptic targeting of excitatory neurons by interneurons has
been observed
8
, the general strategy of inhibitory synaptic
connectivity is less clear. Some studies show a dense,
nonspecific inhibitory connectivity between interneurons and
nearby excitatory neurons
9–12
, whereas others reveal a fine-scale
specificity in inhibitory synaptic connections. For example,
fast-spiking (FS) interneurons in layer 2/3 connect prefere-
ntially to neighbouring excitatory neurons that form reciprocal
connections with them
13
. Similarly, layer 5 inhibitory inter-
neurons form distinct intralaminar and interlaminar subnetworks
with excitatory neurons
14
. Cholecystokinin-containing basket
cells select their postsynaptic targets based on the long-range
axonal projection pattern of the principal excitatory neurons
15
.
Meanwhile, inhibitory synaptic inputs to pyramidal neurons
exhibit a broad stereotypical spatial pattern across different
neocortical areas
16
. Synaptic connections and network intera-
ctions between different classes of neocortical interneurons also
exhibit a remarkable degree of specificity
17–19
. These studies
suggest a high degree of spatial and functional organization of
neocortical inhibitory interneurons. Notably, interneurons in the
neocortex form highly selective gap junctions (that is, electrical
synapses) with each other, largely based on the interneuron
subtypes
20–25
. Thus, as the specificity of synaptic connections
between excitatory neurons forms the basis for canonical
neocortical circuits, these observations clearly emphasize the
necessity of understanding the connectivity patterns of
neocortical interneurons and, more importantly, the
mechanisms that regulate the assembly of specific inhibitory
microcircuits in the neocortex.
The rich variety of synaptic inhibition in the neocortex is
achieved through diverse subtypes of GABAergic interneurons
that have distinct morphologies, biochemical constituents,
biophysical properties or synaptic connectivity patterns
26–28
.
Previous genetic mapping studies demonstrate that neocortical
GABAergic interneurons are primarily generated in the ventral
telencephalon and migrate tangentially over long distances to the
neocortex
29–37
. Moreover, the spatial and temporal origins of
neocortical interneurons contribute to the specification and
distribution of different subtypes. More than 70% of neocortical
interneurons, including those expressing parvalbumin (PV)
and somatostatin (SST), arise from the progenitors in the
medial ganglionic eminence (MGE) and the preoptic area
(PoA) that express the homeodomain transcription factor
NKX2.1 (refs 33,38–40). The remaining 20–30% of neocortical
interneurons, such as those expressing vasoactive intestinal
peptide and cholecystokinin, are mostly generated in the caudal
ganglionic eminence (CGE)
41–43
. Notably, previous studies
suggest that neocortical interneurons originating from sparsely
labelled dividing radial glial progenitors (RGPs) in the MGE
and PoA (MGE/PoA) frequently form local intralaminar or
interlaminar clusters in the neocortex
44,45
. While this view
had been challenged
46,47
, in-depth analysis demonstrates
that spatial clustering is a reliable feature of clonally related,
MGE/PoA-derived interneurons in the forebrain including the
cortex, hippocampus, striatum and globus pallidus
48
or in the
cortex only (see Results). These findings raise the intriguing
possibility that progenitor origin and lineage relationship
may influence the structural as well as functional organization
of neocortical inhibitory interneurons.
In this study, we investigated the synaptic connectivity of
sparsely labelled neocortical interneurons in clusters originating
from low-titre retrovirus-infected RGPs in the MGE/PoA with a
high probability of being clonally related. Our data suggest that
progenitor origin and lineage relationship influence precise
synapse formation and functional organization of inhibitory
interneurons in the mammalian neocortex.
Results
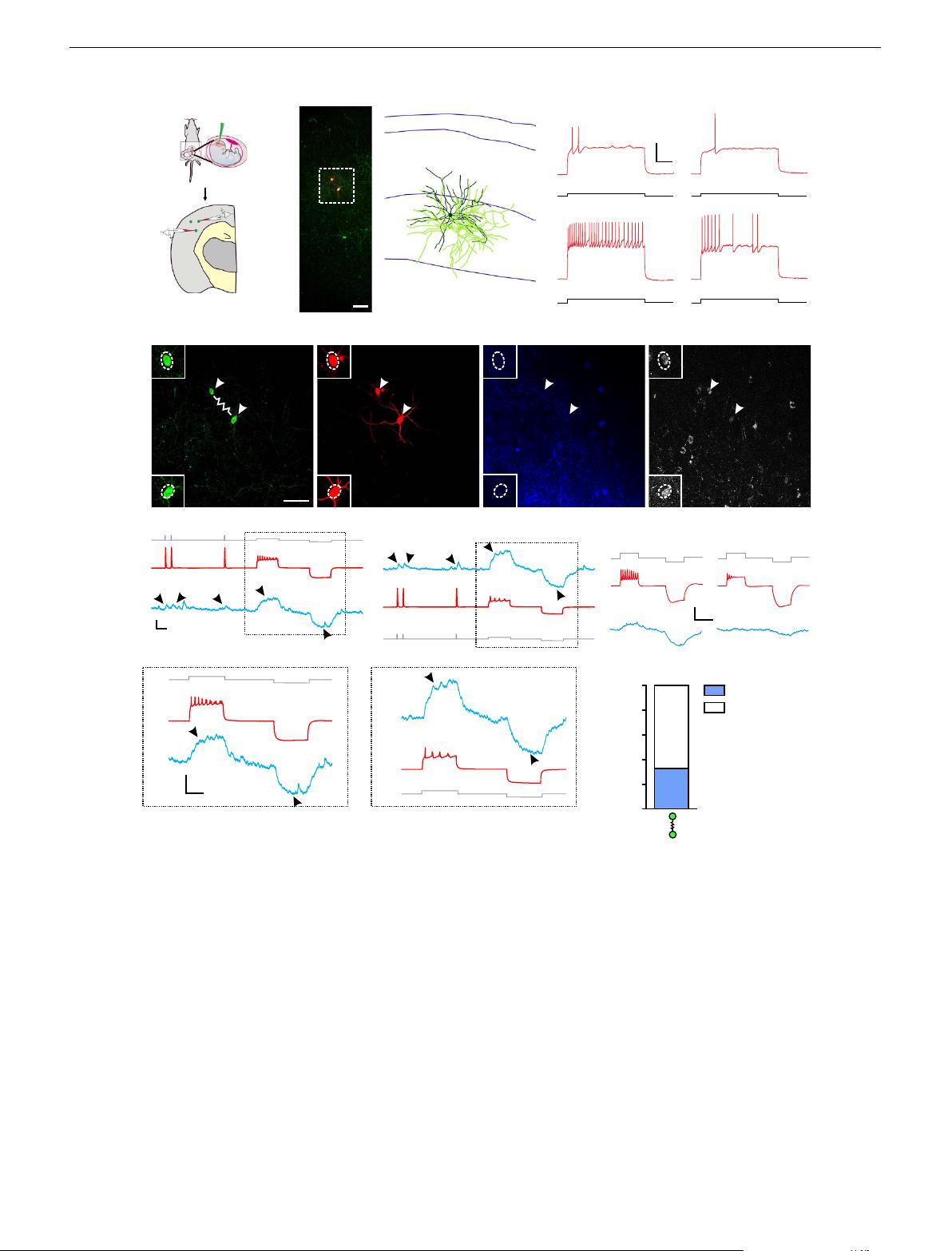
Development of sparsely labelled interneuron clusters.We
previously established a stringent method for selectively labelling
mitotic RGPs at the ventricular zone surface of the MGE/PoA
that predominantly produce neocortical interneurons
44
.By
crossing the Nkx2.1-Cre mice
38
with the LSL-R26
TVAiLacZ
mice
49
, we generated the Nkx2.1-Cre;LSL-R26
TVAiLacZ
mice, in
which the avian tumour virus receptor A (TVA) was specifically
expressed in RGPs of the MGE/PoA (Fig. 1a). To sparsely label
dividing RGPs and their progeny (that is, interneuron clones), we
performed in utero intraventricular injection of a serially diluted,
low-titre avian sarcoma-leukosis virus long terminal repeat with a
splice acceptor (RCAS) expressing enhanced green fluorescence
protein (EGFP) at embryonic day 12 (E12), around the period of
peak neurogenesis in the MGE/PoA
35
. As shown previously
44
,we
observed EGFP-expressing interneurons with characteristic
morphology in the postnatal neocortex (Fig. 1b,c). Moreover,
these neocortical interneurons labelled at a very low density
(that is, on average o10 labelled interneurons in total across the
entire cortical area per 300–400-mm-thick brain slice) frequently
formed spatially isolated clusters across different laminae
(Fig. 1b) or within the same lamina (Supplementary Fig. 5a).
A similar observation of spatial clustering of sparsely labelled
neocortical interneurons arising from dividing RGPs in the
MGE/PoA was also reported in other studies using a distinct or
related method of labelling, including the barcoded retrovirus
library labelling with presumably a single-cell resolution of
clonal identity
45–48
. Our analysis of the two barcoded data
sets
46,47
explicitly demonstrates that the average intraclonal
distance is highly significantly shorter than the average
interclonal distance for the labelled forebrain interneuron
clones in the cortex, hippocampus, striatum and globus
pallidus, suggesting a spatial clustering of clonally related
interneurons in the forebrain
48
. Notably, in the recent Matters
Arising Response paper, Mayer et al.
50
stated that ‘clonally related
cortical interneurons are no more clustered than interneurons
that are not lineally related’ based on a lack of statistical
significance in the comparison of the intra- and interclonal
distances of the labelled cortical-only interneuron clones in the
barcoded data set
50
. However, this lack of statistical significance is
likely due to an insufficient sampling of the study (n ¼ 3 brains).
Should one include the other single barcoded data set labelled
with the same method and analysed in a similar manner
47
(that
is, combining the two barcoded data sets), the average intraclonal
ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/ncomms16091
2 NATURE COMMUNICATIONS | 8:16091 | DOI: 10.1038/ncomms16091 | www.nature.com/naturecommunications



 我的内容管理
展开
我的内容管理
展开
 我的资源
快来上传第一个资源
我的资源
快来上传第一个资源
 我的收益 登录查看自己的收益
我的收益 登录查看自己的收益 我的积分
登录查看自己的积分
我的积分
登录查看自己的积分
 我的C币
登录后查看C币余额
我的C币
登录后查看C币余额
 我的收藏
我的收藏  我的下载
我的下载  下载帮助
下载帮助 
 前往需求广场,查看用户热搜
前往需求广场,查看用户热搜

 信息提交成功
信息提交成功