
2
Annals of the New York Academy of Sciences
medical need, a clear rationale for application in
disease indications, bioavailability, appropriate phar-
macokinetics and pharmacodynamics, efficacy and
safety, a therapeuticwindow, asynthetic route that can
be operated on a large scale, a competitive advantage
over current and emerging therapies, biomarkers, and
aclearmechanisticproofofconcept,justtonameafew.
After the advances in assay development and au-
tomation technologies during the 1990s, more than
200,000 compounds can be tested in so-called HTS
factories per day. With compound archives of large
pharmaceutical companies containing more than
1,000,000 compounds, speedin HTSis nota criterion
in lead discovery anymore. What has not lived up to
the expectations is the gain inproductivity of this drug
discovery process and the success rate measured in
terms of new drug approvals. Despite ever-increasing
researchanddevelopmentinvestments,thenewmolec-
ular entities, that is, the number of new U.S. Food and
DrugAdministrationdrugapprovalsperyear,hasbeen
decreasing since 1996.
1
The high attrition rates dur-
ing later stages of clinical compound testing have also
contributed to the widely lamented productivity crises
in drug discovery.
2–5
To bring more and better new
molecular entities in a shorter time and with less in-
vestment to the market, companies focus strongly on
the reduction of attrition rates during later stages of
drug development. The strategy to achieve this goal is
toproduce higher-quality leadcompounds for promis-
ing targets at the beginning of the process by setting
predefined criteria for lead optimization and by plan-
ning the clinical trials as early as possible.
Within the highest-ranked strategicelementsforim-
proving drug discovery productivity are the following:
•
Functionalizing the human genome by novel
molecular pathway approaches, by introducing
new in vivo genetic models, and by expanded
genome mining.
6
•
Increasing the understanding of disease-
underlyingmechanismsto choosevaliddrug tar-
gets asstarting points.Translational biology and
translationalmedicinearoseasnewscientificdis-
ciplines and are indicative for the integration of
clinicalscience into theearlydrugdiscovery pro-
cess. Proof-of-concept trials and patient stratifi-
cation are equally important.
•
Findingwaystoexpandthechemicaluniverseby
increasinglibrarydiversity andimprovements in
structural biology and modeling.
•
Finally, there is a need for more quantitative bi-
ology in lead discovery. Functional and mech-
anistic characterization of hit compound–target
protein interactions by biophysical methods is
an active field of research. Currently there is
much hype throughout the pharmaceutical and
biotechnology industry about using artifact-free
detection technologies. It is generally assumed
thatlabel-freedetectionislessinfluencedbymea-
suring artifacts than fluorescence detection.
Changing Paradigm in Detection
Technologies for Drug Discovery?
Until about 1990, radioactivity was the detection
technology of choice for assays and early screening
methods; today it is almost only used for scintillation
proximity assays in HTS. In the period between 1990
and 2005 HTS was strongly dominated by fluores-
cencespectroscopyalmostexclusivelyusedasdetection
method. Later fluorescence imaging for high-content
screening became increasingly important. Label-free
detection really started to kick in around 2005 and
beyond with calorimetricmethods, such as differential
scanning calorimetry (DSC) and isothermal titration
calorimetry (ITC); biosensor techniques on plates and
chips; and, most importantly, mass spectrometry (MS)
applied to screening, imaging, and profiling. Although
the label-free methods hold great promise for future
applications in applied science, a current comparison
ofadvantagesanddisadvantagesoffluorescencedetec-
tion versus label-freedetection methodsrevealsa clear
strategy for method selection.
The major benefit of any label-free detection tech-
nique is the lack of a sensor or tracer linked to the
receptor or ligand. The usual concern is that a la-
bel might influence the molecular recognition event.
With appropriate care taken by the scientist to per-
form all necessary controls and by keeping in mind
that the fluorescently labeled ligand is nothing more
than a tool for competition screening, most fluores-
cence assaytechniques will deliver reliable results. MS
detection is currently successfully applied for complex
enzymatic screens, such as lipid metabolic assays with
mass changes involved. Such assays are sometimes dif-
ficult to run with fluorescence detection. ITC, DSC,
andlight-scatteringmethodsstarttosubstantiallyaffect
lead optimization and triaging of hit lists. Only multi-
dimensionalfluorescencefluctuationanalysis atsingle-
moleculeresolutionappliedincompetitionstudieswith
alabeledsurrogateligandandaseries ofinhibitorscan
provide better results. Currently, fluorescence meth-
ods are superior to label-free detection techniques be-
cause of their high sensitivity down to single-molecule
and single-cell resolution. Together with the possibil-
ity to measure ligand binding in homogeneous mix-
tures at equilibrium, fluorescence techniques are su-
perior in delivering quantitative thermodynamic and
kinetic data (dissociation constants, rate constants of




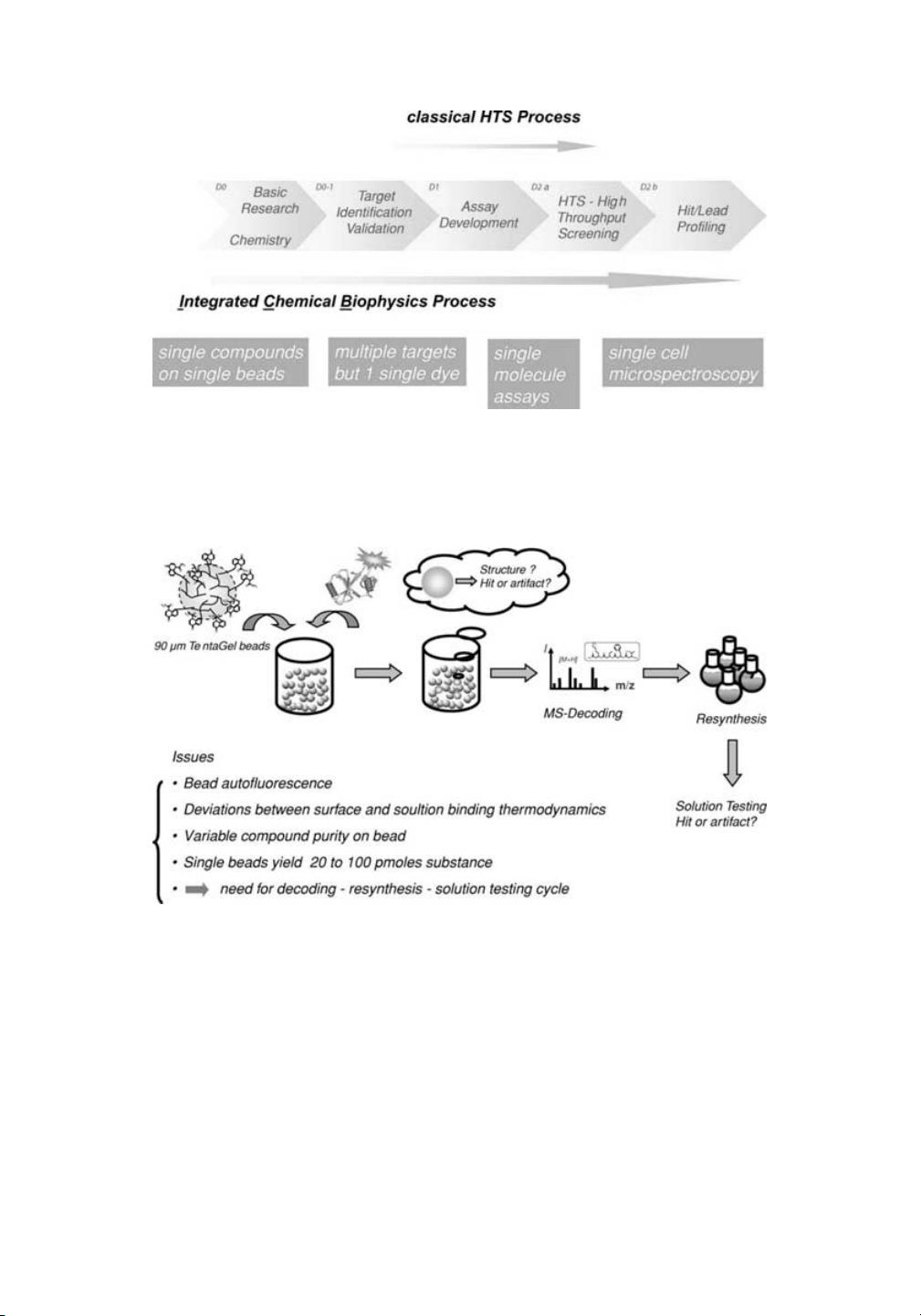

 我的内容管理
展开
我的内容管理
展开
 我的资源
快来上传第一个资源
我的资源
快来上传第一个资源
 我的收益 登录查看自己的收益
我的收益 登录查看自己的收益 我的积分
登录查看自己的积分
我的积分
登录查看自己的积分
 我的C币
登录后查看C币余额
我的C币
登录后查看C币余额
 我的收藏
我的收藏  我的下载
我的下载  下载帮助
下载帮助 
 前往需求广场,查看用户热搜
前往需求广场,查看用户热搜

 信息提交成功
信息提交成功