INHIBITION OF METOPROLOL METABOLISM BY AMINO ACIDS IN PERFUSED
RAT LIVERS
Insights into the Food Effect?
BO WANG AND HUGH A. SEMPLE
College of Pharmacy and Nutrition, University of Saskatchewan
(Received June 18, 1996; accepted December 10, 1996)
ABSTRACT:
A mixture of amino acids inhibits propranolol metabolism in per-
fused rat livers. To obtain mechanistic information about the inter-
action, a related but less tissue-bound drug, metoprolol, was used
to determine V
max
and K
M
for parent drug and two metabolites in
the presence and absence of amino acids. Six groups of 4 livers
from 24 male Sprague-Dawley rats were perfused in the single-
pass mode at 3 ml/min/g liver for 130 min with oxygenated buffer
containing 3.74, 4.49, 5.61, 7.48, 18.7, or 44.9
m
M metoprolol. From
50 to 90 min, a balanced amino acid mixture was included in the
buffer. Samples of liver effluent taken every 5 min were analyzed by
HPLC for metoprolol and two metabolites,
a
-hydroxymetoprolol
and O-demethylmetoprolol. Steady-state concentrations of drug
determined before, during, and after amino acids were used to
determine V
max
and apparent K
M
values by nonlinear curve-fitting
under each condition. Amino acids reversibly reduced the V
max
values of metoprolol and both metabolites by ;50% without sig-
nificantly affecting apparent K
M
values. As a result, large increases
in availability occurred, especially at low metoprolol inlet concen-
trations (>90%). Amino acids also increased oxygen consumption
until the effluent buffer was almost depleted. Possible mechanisms
influencing V
max
include direct inhibition of metabolic enzymes by
amino acids or cosubstrate (NADPH or oxygen) limitation. Amino
acid-mediated pericentral oxygen depletion in the hepatic sinu-
soids could result in inhibition of drug-metabolizing enzymes, and
is consistent with a reduction of V
max
and oxygen depletion in the
effluent buffer during amino acid coinfusion. We postulate that one
or more of these mechanisms could contribute to the interaction
between food and high first-pass drugs observed in humans.
Rac-metoprolol is one of several drugs known to exhibit increased
oral availability when coadministered with a high-protein meal, in-
cluding propranolol, propafenone, labetolol, zuclopenthixol, and
dixyrazine (1). Although this food interaction was first observed two
decades ago, its mechanism remains to be fully elucidated, despite
intensive study (1). The mechanism is likely to be complex, with
contributions from more than one of the physiological responses to
food combining to cause a net increase in AUC
oral
.
1
Because drugs
that show the “food effect” are almost completely absorbed from the
gastrointestinal tract, it is generally agreed that the interaction is due
to a reduction in first-pass metabolism (1). Studies to date on pro-
pranolol have concentrated on the liver, although recent studies in
rabbits suggest the possible contribution of intestinal metabolism (2).
A reduction in hepatic first-pass metabolism could occur through
changes to hepatic blood flow, plasma protein binding, or metabolic
activity. The hypothesis that the increase in AUC
oral
of propranolol
could be caused by a transient increase in hepatic blood flow (3) was
questioned, because flow changes could not account for the magnitude
of the increase (4, 5). It is also unlikely that food causes an increase
in the unbound fraction of propranolol in plasma (6). Experiments in
humans to test for transient changes in metabolic activity have shown
that food causes inhibition of presystemic primary conjugation of
propranolol (7), although data relating to the more important phase I
pathways are inconclusive (8, 9). Nevertheless, simulations have
indicated that propranolol availability would be most sensitive to
changes in apparent V
max
and somewhat sensitive to changes in
apparent K
M
, both of which contribute to intrinsic clearance (10).
To explore further whether nutrients could inhibit propranolol
metabolism, amino acids were coinfused into rat livers perfused with
buffer containing propranolol (11). Global inhibition of metabolism
was observed, indicating that at concentrations achieved after a high
protein meal, amino acids could inhibit all of the pathways of pro-
pranolol metabolism and that the degree of metabolic inhibition was
related to the concentration of amino acids in the buffer. Hepatic
tissue binding prevented further exploration of the mechanism using
propranolol as a model drug. Pilot studies showed, however, that
metoprolol is much less extensively tissue bound and its metabolism
was inhibited by amino acids, making it an appropriate model drug for
mechanistic studies involving the measurement of Michaelis-Menten
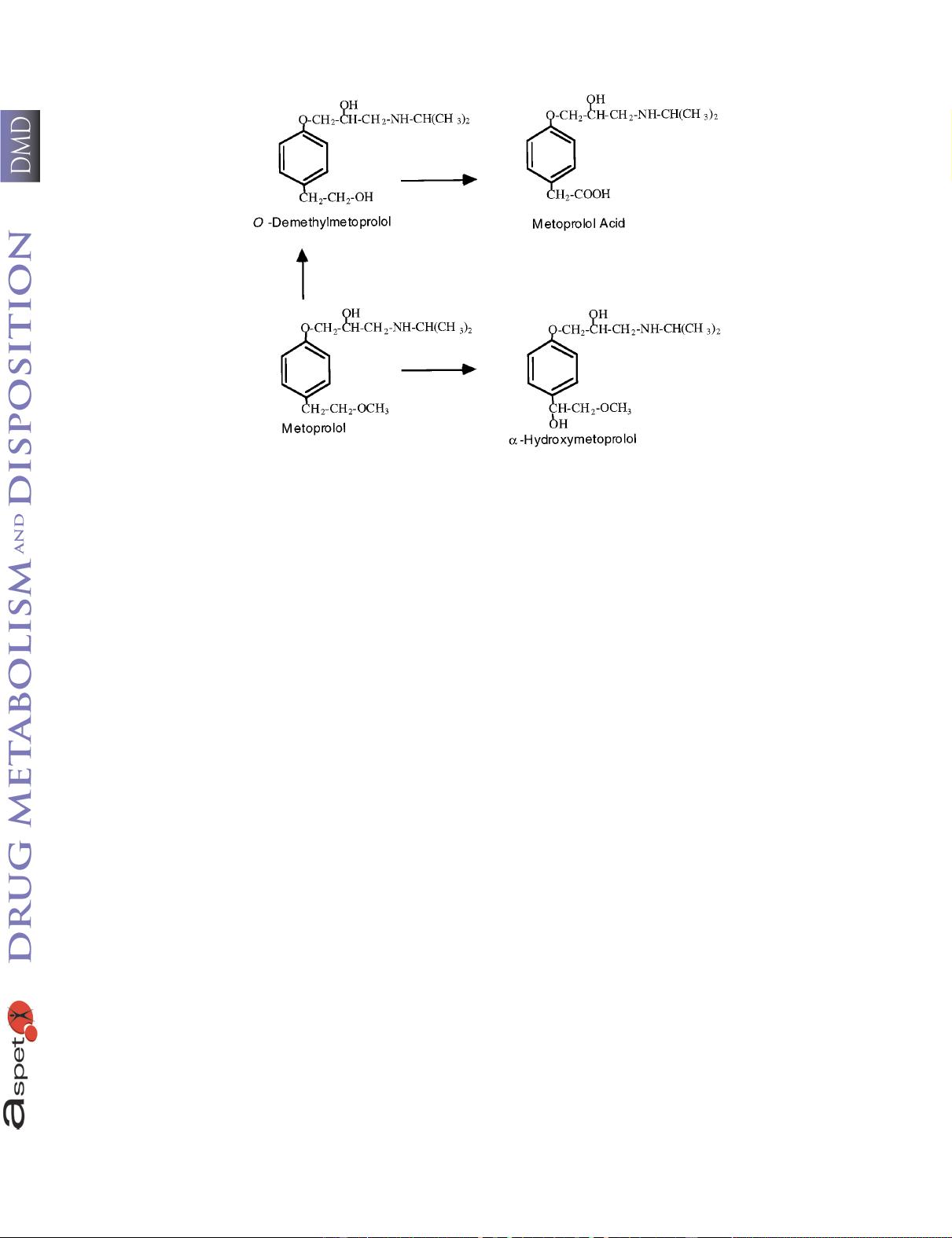
parameters. The metabolism of metoprolol is depicted in fig. 1. We
describe herein experiments that confirm our pilot observations in
perfused rat livers and that focus on amino acid effects on the apparent
V
max
and K
M
of metoprolol metabolism. As part of the liver viability
assessment, effluent buffer O
2
content was measured.
1
Abbreviations used are: AUC, area under the plasma concentration-time
curve; O
2
, oxygen; OH, hydroxy; C
ss
, concentration at steady-state; t
ss
, time to
steady-state; C
in
, inlet concentration; C
out
, outlet concentration; Q, buffer flow
rate; E, organ extraction ratio; CL, organ clearance; F, organ availability;
n
,
velocity of metabolism; C
out,m
, outlet concentration of metabolite; [S], substrate
concentration; MW, molecular weight; CYP, cytochrome P450.
This study was supported by the Medical Research Council of Canada, De-
velopment Grant DG-405 and by the Heart and Stroke Foundation of Saskatchewan.
This work has been presented in part as an abstract [ISSX Proc. 8, 311 (1995)].
Send reprint requests to: Dr. Hugh A. Semple, College of Pharmacy and
Nutrition, University of Saskatchewan, 110 Science Place, Saskatoon,
Saskatchewan, Canada S7N 5C9.
0090-9556/97/2503-0287–295$02.00/0
D
RUG METABOLISM AND DISPOSITION Vol. 25, No. 3
Copyright © 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in U.S.A.
287
at NSTL on June 16, 2012dmd.aspetjournals.orgDownloaded from


 我的内容管理
展开
我的内容管理
展开
 我的资源
快来上传第一个资源
我的资源
快来上传第一个资源
 我的收益 登录查看自己的收益
我的收益 登录查看自己的收益 我的积分
登录查看自己的积分
我的积分
登录查看自己的积分
 我的C币
登录后查看C币余额
我的C币
登录后查看C币余额
 我的收藏
我的收藏  我的下载
我的下载  下载帮助
下载帮助 
 前往需求广场,查看用户热搜
前往需求广场,查看用户热搜

 信息提交成功
信息提交成功