
optimized at the restricted Hartree−Fock (HF) level of the
quantum chemical theory using the minimal STO-3G basis sets.
On the basis of optimized geometries of the 3D structure,
NMR was calculated using density functional theory (DFT)
PBE1PBE exchange correlation functional and 4-31G basis sets.
This work provided a general methodology to develop a 3D
model based on a known 2D model of kerogen. Li and co-
workers
77
has defined the lowest energy conformation of the
Huadian kerogen molecular structure using a simulated
annealing procedure. Structu ral parameters of the lowest
energy conformation were further analyzed. On the basis of
the above molecular dynamics simulation met hods, they
proposed the possible reaction sites and pyrolysis process of
kerogen.
For the 3D molecular simulation, the interaction between
kerogen and other molecular units coming from the organic
matter of oil shale should also be considered. In general, the
organic matter mainly composes of kerogen (the most
abundant), asphaltenes, resins, hydrocarbons, and other fluids
(such as carbon dioxide, water, and nitrogen). Galliero et al.
78
constructed a 3D molecular model of organic matter presenting
a type II oil shale in the middle of the oil generation window
using molecular dynamics simulations (NPT molecular
dynamics and force fields). Their results provide a lot of
valuable information at the molecular levels, such as the fluid
distribution within the organic matter, pore size distributions,
isothermal compressibility, and dynamic of the fluids within the
kerogen matrix. Very recently, a molecular dynamic simu-
lation
79
has been applied to a representative set of kerogen for
the purpose of obtaining quantitative predictions of volumetric
properties, which also take into account the interaction among
them. The density results are well in agreement with the well-
documented trends of kerogen density with thermal maturity
and organic type.
As mentioned above, both Orendt and Li adopted a
simulated annealing algorithm to seek the lowest energy
conformation of kerogen. However, the simulated annealing
algorithm
80
is based on a given 2D model with fi xed
connections and structural characteristics to obtain a more
compact and superior conformation than the initial model.
That is, molecular dynamic simulation considers the conforma-
tional isomer rather than the geometric isomer. The kerogen
model has numerous geometric isomers. Only considering one
connection case to seek the lowest conformation is not
convincing and sufficient. Besides, it is impracticable to
endlessly optimize all possible geometric isomers for a large-
sized kerogen molecule because of limitations in computational
resources.
80−82
Thus, it is valuable to explore the relationship
between isomer stability and molecular geometries of kerogen.
The HF method provided a reasonable starting point to
probe the chemical structure for macromolecular systems but
failed to consider the electron correlation effects, which is
important for calculating structure and energies of a molecule.
To overcome the weakness in electron correlation effects,
coupled cluster, configuration interaction, and perturbation
theory have been developed. However, they are not suitable for
macromolecular systems because of the high computational
cost. In contrast, the DFT method has been proven to be
efficient in evaluation of the physicochemical property for a
wide range of compounds with a large size because of proper
consideration of electron correlation and moderate computa-
tional cost. Currently, DFT is a widespread acceptance method
for understanding physicochemical properties of macromolec-
ular systems. It should be stressed that the DFT method turned
out to be insufficient for describing the weak intra- or
intermolecular interactions, but it is the most accurate method
thatcanbeusedonlargemoleculesatanaffordable
computational cost.
During the past few years, our group did research in the
combustion characteristics and physicochemical properties of
oil shales, combustion and co-combustion characteristics of
semi-coke, etc.
83−99
Semi-coke mentioned here refers to the
solid waste left after oil shale retorting, which contains phenols,
polycyclic aromatic hydrocarbons (PAHs), and oil prod-
ucts.
100,101
However, few studies were focused on the chemical
structure of kerogen in oil shale. Therefore, the aim of this
paper is (i) to obtain the detailed structural information on
Huadian kerogen through a variety of experimental methods,
(ii) and on the basis of these experimental data, a series of
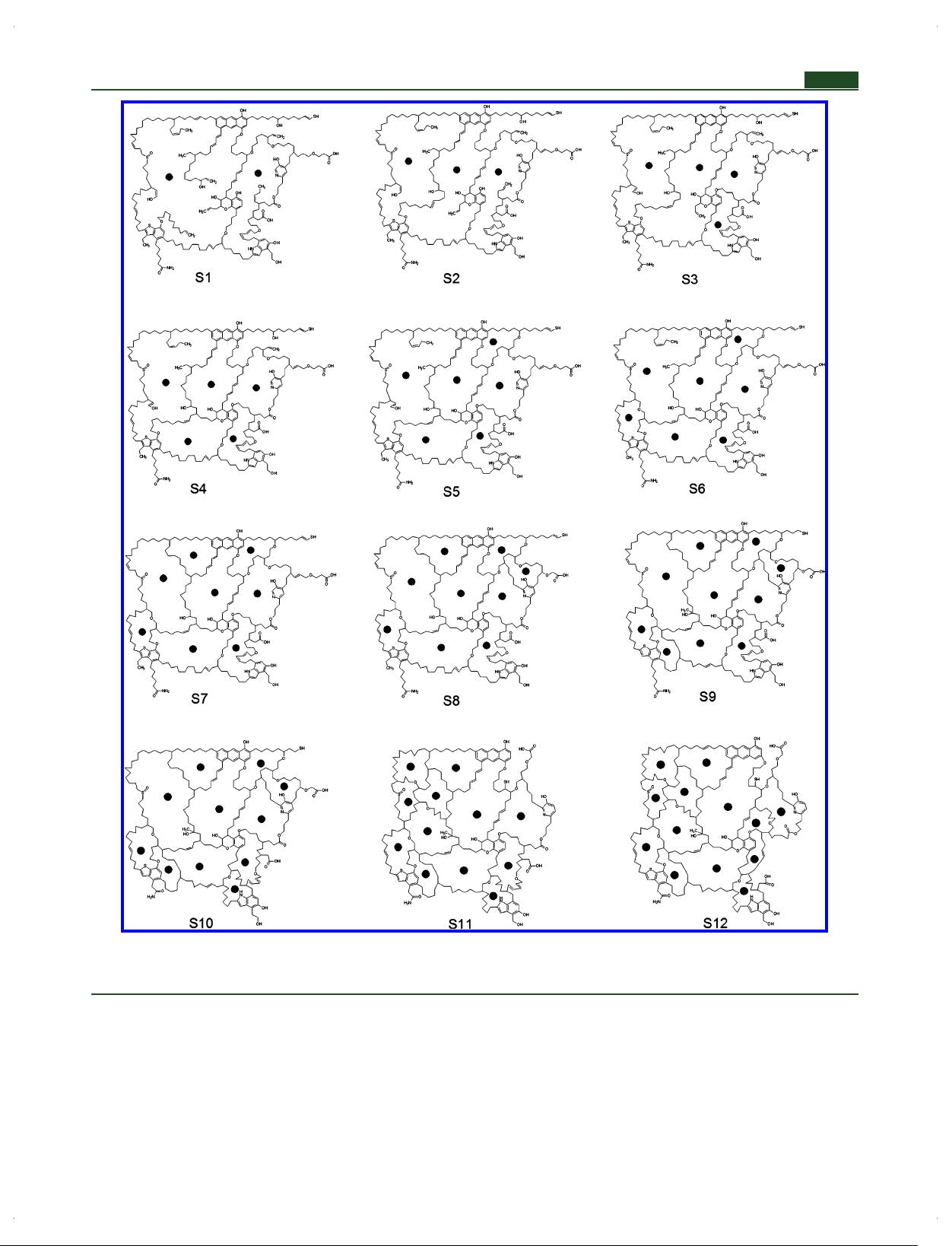
Huadian kerogen 3D isomer models have been constructed to
consider the carbon skeleton isomerization, the substituted
position effects of the aromatic ring, aliphatic ether bond,
carboxylic acid, and carboxylic acid derivative, and the quantity
of tertiary and quaternary carbons on model stability using
DFT calculations.
Research in this paper probably provides a scientific guide to
build and find the kerogen 3D model. Although the constructed
kerogen model in this paper is not the lowest energy
conformation among infinite kerogen geometric isomers, it is
still valuable for building the model of kerogen. What we
emphasize is that, during the process of building a model of
kerogen, the carbon skeleton isomerization, substituted
position effects, and quantity of tertiary and quaternary carbons
should be taken into reasonable considerations. For most of the
cases in the current, these factors were not considered or
inadequately described in building a 3D model of kerogen.
2. EXPERIMENTAL SECTION
2.1. Sample Preparation. The oil shale sample used in this paper
was obtained from Huadian mine located in Jilin, China. Large oil
shale blocks were first crushed and sieved to 0.2 mm to obtain the
experimental oil shale sample. Bitumen was extracted from the shale
using chloroform. Then, the oil shale sample was demineralized by a
four-step extraction procedure using HCl−HF−HNO
3
−HCl. The
demineralization effect has been checked using the X-ray diffraction
(XRD) technique (see below).
The results of elemental analysis of Huadian kerogen are shown in
Table 1. According to the atomic H/C and O/C ratios,
51,102
Huadian
kerogen belongs to type I, although the atomic H/C and O/C ratios of
our kerogen differ from other Huadian kerogen reported in previous
studies.
23,77
This may be due to the fact that the reported sample
preparation process did not remove asphaltenes. The various structure
of kerogen obtained in the same mine but different sedimentary layers
Table 1. Elemental Analysis of Huadian Kerogen (wt %, Dry
and Ash-Free Basis)
C 71.73
H 8.885
O 11.033
N 1.29
S
t
a
2.257
H/C
b
1.486
O/C
b
0.115
N/C
b
0.189
S/C
b
0.012
a
Total sulfur.
b
Atomic ratio.
Energy & Fuels Article
DOI: 10.1021/ef502759q
Energy Fuels 2015, 29, 4122−4136
4123



 我的内容管理
展开
我的内容管理
展开
 我的资源
快来上传第一个资源
我的资源
快来上传第一个资源
 我的收益 登录查看自己的收益
我的收益 登录查看自己的收益 我的积分
登录查看自己的积分
我的积分
登录查看自己的积分
 我的C币
登录后查看C币余额
我的C币
登录后查看C币余额
 我的收藏
我的收藏  我的下载
我的下载  下载帮助
下载帮助 
 前往需求广场,查看用户热搜
前往需求广场,查看用户热搜

 信息提交成功
信息提交成功